Preface
The promise of digital health solutions has been a long time coming. Different applications, websites and portals and traditional ICT have all been viewed as saviours of healthcare. And the Covid crisis has increased this pressure to find an answer. However, healthcare is a complex ecosystem without silver-bullet solutions. Digital health can reduce healthcare costs, improve patient care and provide new business opportunities, but it can only do it as an integral part of health and healthcare.
The spearhead of digital health is digital therapeutics (DTx): evidence-based software to treat, manage or prevent diseases or conditions. They are analogous to drugs in that they are medical treatments, need to be backed by clinical evidence and provide meaningful results. The requirement for meaningful results or positive healthcare effects aligns DTx closely to treatments and healthcare. To realise the potential of DTx, countries need to foster innovative companies, build transparent pathways to market and deployment and to integrate DTx as part of care pathways.
The focus in this paper is on the systemic perspective, including strategic frameworks, assessment at national or regional level, reimbursement, and deployment and integration of DTx into healthcare pathways.
This paper examines approaches related to digital therapeutics by five European countries or regions: France, Belgium, Germany, England and Catalonia. We examine how DTx solutions are defined, whether DTx are included in overarching strategic digital health policies, how the assessment of the solutions is performed, what the reimbursement and prescription pathways are, and how the data is integrated and used for secondary purposes. Finally, we propose steps for European alignment and a generic approach for European countries.
With this working paper we aim to add to the knowledge about digital therapeutics among European stakeholders. The work will continue into late 2021 with another publication that will focus on what should be done in Finland in this novel field of healthcare. Throughout 2021, we have been having dialogue with stakeholders about the readiness to pilot DTx in care pathways, and if the reception proves to be positive, we will launch a call for pilot schemes in early 2022.
Currently, Sitra focuses on three thematic areas: sustainability solutions, the fair data economy, and democracy and participation. Within the fair data economy theme, health is one of sectors in which we operate.
We collaborate with partners from different sectors to research, trial and implement bold new ideas that shape the future. The collaboration also leads to a continuous dialogue about the challenges and opportunities within our selected thematic areas and means we can hear proposals from partners about where Sitra’s intervention would have most impact. It was during this dialogue in early 2021 that digital therapeutics was first mentioned by several partners from the private sector as one area to study.
We hope you find this working paper insightful and we thank everyone who has taken part in its creation. Special thanks to i-HD and Empirica, who helped put together the research and conducted Europe-wide interviews.
24.11.2021
MARKUS KALLIOLA
Project Director, Health data 2030, Sitra
Summary
Digital therapeutics (DTx) are medical interventions delivered directly to patients using evidence-based, clinically evaluated software to treat, manage and prevent a broad spectrum of diseases and disorders. They often rely on behavioural and lifestyle changes or are applied in combination with conventional medical treatments such as drugs or radiation therapy. They are often used for untreated or undertreated conditions such as diabetes, insomnia or anxiety.
DTx have the potential to help patients, clinicians, businesses and healthcare systems. The industry is growing and offers business potential for pharmaceutical companies and start-ups. Clinicians can benefit by spending less time on non-productive work and using automated triggers for intervention. Patients can take an active role in their health instead of being passive recipients of care. For healthcare systems, DTx can reduce costs and provide better value.
In the five European countries or regions selected for this study (France, Germany, England, Belgium and Catalonia) the criteria for assessing digital health solutions are mostly the same. They all use different terminology and draw the lines in different places, but the fundamental criteria are similar, including safety, usability, clinical evidence, socio-economic effectiveness and level of integration into current ways of working. The process or pathway to market for DTx companies differs. Some countries build their approach on conventional procurement whereas others provide reimbursement for any solutions that fulfil the criteria. DTx (and other analogous categories) is a relatively new subject areas, and the approaches to it are still taking form.
We recommend that Europe start building a single market for DTx. While health systems have fundamental differences, the requirements for digital health solutions are the same to a large extent. At a minimum, clinical evidence from one country should be recognised in another country.
European countries should build inclusive national strategies that address DTx explicitly. Clear and transparent certification pathways should be created. Certification is not enough, and market access should also be designed so that it facilitates innovation in both business and the healthcare system. To avoid DTx becoming isolated and sporadic solutions, access to real-world data should be facilitated as much as possible. National approaches should consider that implementation of DTx is a major cultural change for healthcare. Resistance to change and integration into healthcare systems should be built into any national approach.
1. Pill or app? New tools for healthcare
Digital Therapeutics (DTx) use software to treat, prevent and manage diseases and conditions. They offer great promise for better care, personalisation, empowerment and lower healthcare costs.
Rate of change in healthcare
Healthcare systems have historically been slow to adopt new technologies. The Covid-19 pandemic has driven the adoption of digital technologies in all sectors and regions. Healthcare is no exception. The rapidly changing operational environment has shifted many physical interactions to digital interfaces but this has not happened without costs. Covid-19 has severely disrupted the prevention and treatment of noncommunicable diseases.
While healthcare is slow to adopt new technologies, consumers have an ever-increasing number of health and wellness apps to choose from. There are over 350,000 health and wellness apps available to consumers with the numbers increasing on average by 250 apps a day in 2020. Disease-specific apps are increasing in number. The amount of apps raises the question of quality. Are these apps helpful for their users? Are users able to assess the quality of these apps?
Five European countries or regions were surveyed for comparison: France, Belgium, England, Germany and Catalonia. These five were selected for being active in the digital health space, each in their own way. At least one public servant and one person from the DTx industry was interviewed. An expert workshop was held to discuss the findings further.
Digital therapeutics: The spearhead of digital health
Within the field of digital health apps and tools lies a subset with a high bar for entry called digital therapeutics (DTx). They are clinically evaluated and prevent, manage or treat specific conditions. There is no unequivocal definition of DTx. Some definitions require randomised controlled trials while others qualify general well-being applications with potentially beneficial health effects. Digital Therapeutics Alliance (PDF) define digital therapeutics as follows: “Digital therapeutics (DTx) deliver medical interventions directly to patients using evidence-based, clinically evaluated software to treat, manage and prevent a broad spectrum of diseases and disorders”.
DTx are medical devices that fall either into the medium to high-risk class (Class II) or low-risk class (Class I), depending on the specifics. A DTx tool for improving insomnia might not have as high a risk class as a DTx treating cancer patients in remission. Both examples exist in the countries surveyed.
DTx often come in the form of applications for mobile devices or web applications but they can also come in other forms, such as wearables or desktop computers. They often make use of behavioural coaching. Some achieve this through gaming. Games can challenge mental capabilities or stimulate damaged areas of the brain. Games are generally currently focused on mental health and musculoskeletal issues.
To illustrate DTx, it might be easier to start by defining what DTx are not. General preventative health applications downloadable on any application marketplace focusing on issues like smoking cessation or weight management are not considered to be DTx. However, this line can sometimes be blurred. A clinically proven smoking cessation application that is prescribed by a clinician for clinically diagnosed disorder can be considered DTx. The key differences are the level of evidence, regulatory oversight and the prescription by a clinician. Self-prescription of DTx is already a reality. As of October 2021, Scotland offers DTx for anxiety and insomnia nationally for the whole population based on self-prescription.
This separation is important for everyone: regulators, clinicians, companies, consumers and patients. For regulators, DTx claims need to be verified (for example, that use shortens hospital stays by 80%), while general health and wellness apps claims do not need validation (for instance, one may claim that it makes people feel less stressed) because one is a medical treatment and a medical device whereas the other is simply an application that can help with stress. Clinicians need to be able to rely on the products or services they prescribe. For companies, it is important to show the effectiveness and excellence of their solutions and products. Finally, for consumers and patients, it is important to distinguish between general well-being and the treatment of a condition.
Table 1: Digital health categories
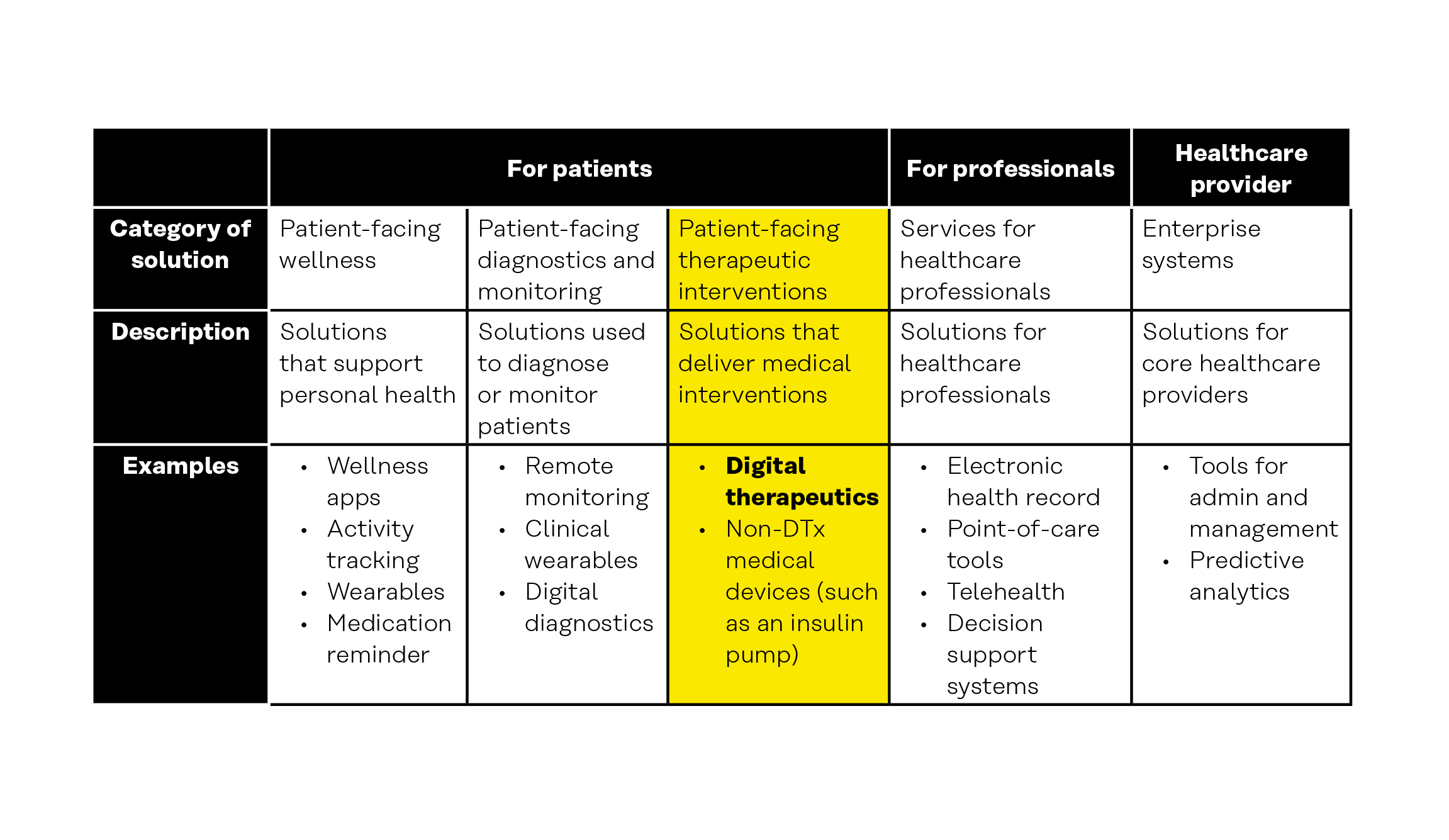
Concepts that are relevant to digital therapeutics include the following.
- Digital health, digital medicine and digital therapeutics (PDF). DTx belongs under the umbrella term “digital health”. Digital health, as the name suggests, includes everything digital that relates to health. It includes concepts such as mobile health (mHealth), telehealth (also known as telemedicine), smart devices and sensors and wearables. Digital health does not require clinical evidence or regulatory oversight. The next subgroup in digital health is digital medicine. Digital medicine requires clinical evidence. DTx is the spearhead of digital health as its tools are both clinically proven and provide real-world results. Digital medicine is located between these two with a requirement for clinical evidence but no requirement for real-world outcomes.
- eHealth is often used interchangeably with digital health. The difference for this discussion is not highly relevant. However, the WHO definition of eHealth (PDF) is contained within digital health. Digital health includes emerging areas such as big data and advanced computing, artificial intelligence and genomics.
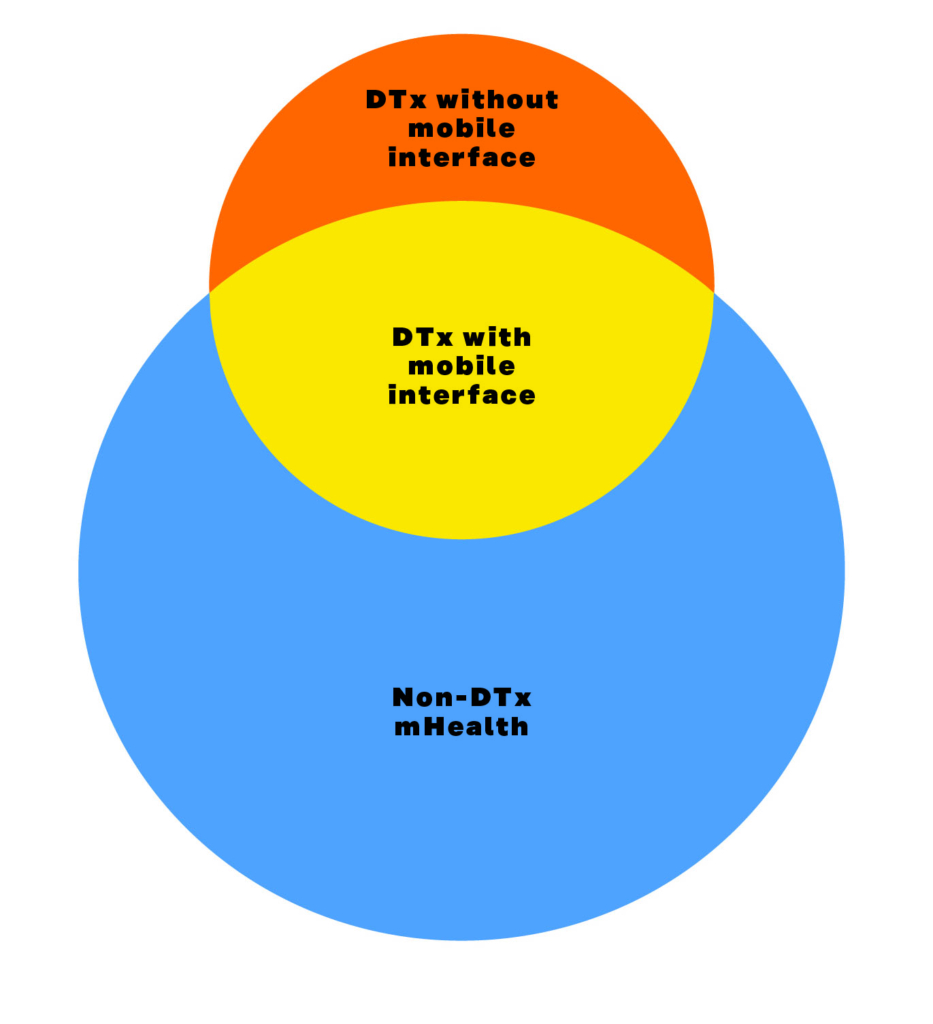
- mHealth is defined as “use of mobile and wireless technologies to support the achievement of health objectives”. mHealth is a term that can contain many DTx but also other mobile-enabled technologies. Currently mobile applications are a key interface for DTx but they can also be delivered by non-mobile technologies. mHealth includes solutions that are not clinically evaluated, do not gather data from their users or are not treatments for medical conditions. The following figure illustrates the relationship between DTx and mHealth. Health apps are applications for mobile devices.
- Telehealth or telemedicine (PDF) refers to the use of telecommunication to provide remote healthcare services. Telehealth sometimes refers to health in broader terms than telemedicine, but this difference is not significant for our purposes. The most common practical example is remote clinician-to-patient interaction. DTx does fulfil the definition of telehealth but telehealth is a broader concept like mHealth.
- Wearables are technologies that that are worn on or close to the surface of the skin. DTx can utilise wearables but most of them currently do not. The most common wearables are smart watches and wrist-worn heart-rate monitors.
Figure 1: Digital health, digital medicine and digital therapeutics.
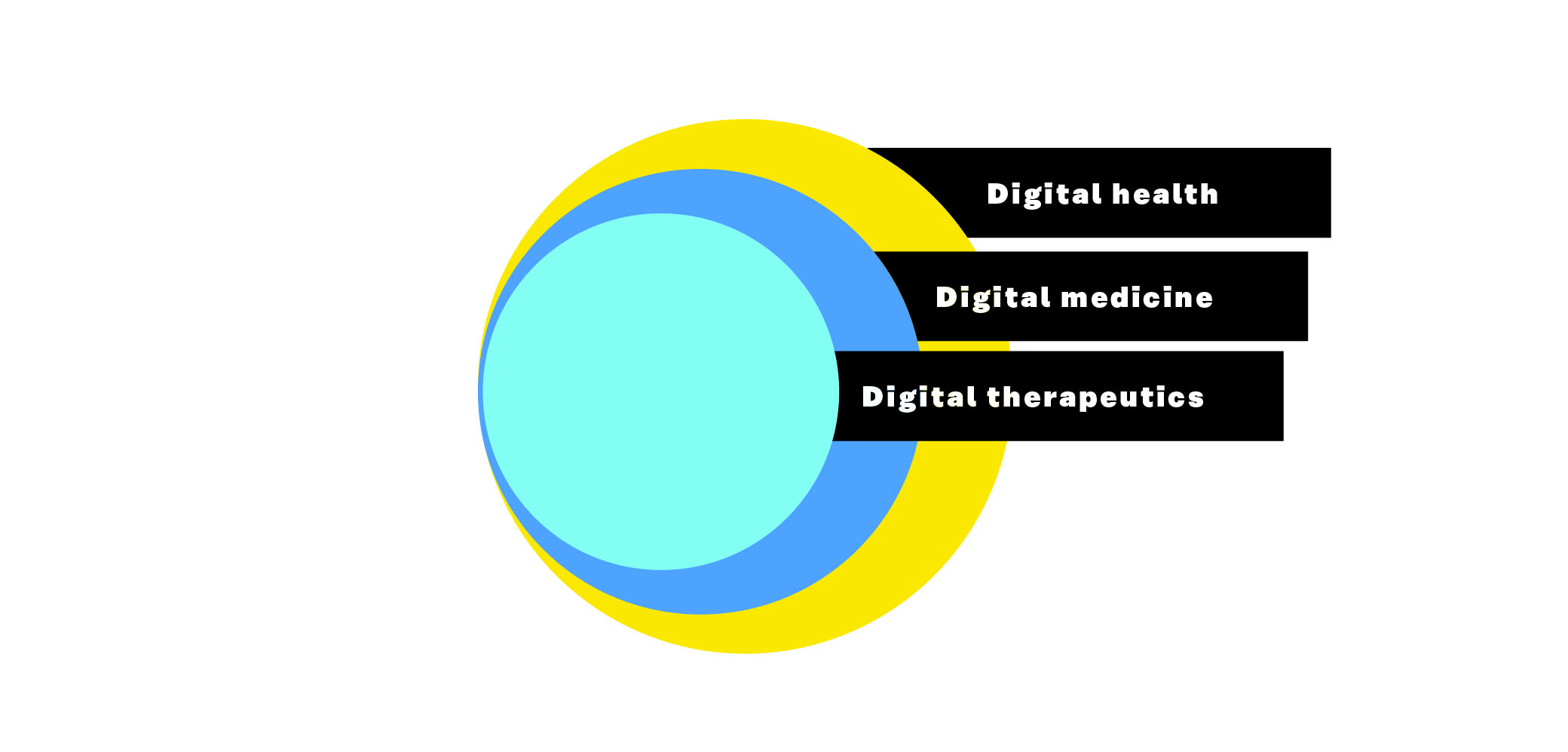
Figure 2: DTx versus mHealth.
The promise of DTx
DTx offers great promise for many mental health conditions, chronic diseases (such as diabetes and cardiovascular diseases) and behavioural changes (like smoking cessation or weight management), to name just a few. For some conditions the benefits materialise when used together with medication, such as cancer treatments, while others can benefit from only the digital solution, such as those with mental conditions. One important example of the potential of DTx is the focus on often poorly addressed conditions and diseases to which conventional medicine offers little help, such as behaviour modification and mental health, including post-traumatic stress disorder (PTSD), substance abuse and insomnia.
In addition to addressing the unmet needs of patients, DTx help to reach patients at home and go beyond the conventional in-person interaction. For example, boosting the effectiveness of conventional cognitive behavioural therapy by offering it digitally in between face-to-face sessions might provide better outcomes. Populations lacking access to one-on-one services can be served in part through DTx.
It is easy to downplay the effects of DTx and digital health solutions in general. However, they do have many tangible positive effects compared to conventional medicine. The positive effects in mental health are more difficult to measure and hard to decipher for ordinary, non-medical individuals. Moovcare offers an example from oncology, a more tangible field of medicine. Their solution has been clinically shown to increase overall survival rates for lung cancer patients in remission by 7.6 months. The work to achieve this and demonstrably prove the effects has taken Moovcare six years. This displays the seriousness of the field. DTx are not nice-to-haves but rather must-haves. The opportunity presented by this field cannot be overstated or missed.
Challenges of DTx
Why have DTx not become a staple of healthcare? Why are pharma companies not investing more in DTx? According to our interviews, there are many reasons, such as resistance to change from doctors, inefficient ways of working, lack of expertise and knowledge, cumbersome regulatory processes and budget constraints. The main hindrances for wider DTx adoption are the lack of integration into health systems and badly aligned incentives. Lack of differentiation from general health and well-being apps has been a challenge for the DTx industry but with the emergence of national frameworks this problem has been lessened, although it has not disappeared completely.
Incentives are poorly aligned for healthcare providers, healthcare payers (those paying for healthcare service or provision through insurance payments or taxation, e.g., insurance company or national government) and companies. With digital health, providers are facing new challenges on top of pre-existing ones. Digital therapies require changes to operations. For instance, data is an important asset, but it requires work to be of use. Healthcare provision is riddled with resource deficiencies. It is no wonder clinicians are not too keen on DTx, as our interviews suggest. DTx should lessen the workload rather than increase it. Paying clinicians per visit might also discourage them from using DTx if it would mean less income from visits.
For payers, the DTx tools need to offer clear improvements economically and to outcomes. Even though value should be the driving force of healthcare, the short-term (within one to two years) economic impact will need to be positive. For instance, healthcare costs caused by smoking materialise in decades, whereas the budgetary time frame is often five years. For countries with single payer or long-lasting memberships, the incentives for DTx should be greater. Consequently, Europe is positioned more favourably in this regard than, for instance, the USA.
2. More investments, better health outcomes and empowerment
DTx offers a multitude of potential benefits for different stakeholders. Countries and Europe in general can attract investments related to DTx. Health systems can benefit from better care, prevention and resource efficiency. Patients can take more control over their health through personalised solutions while healthcare professionals can focus on more value-adding tasks.
More investments for Europe and nationally
Venture capital in the DTx industry increased from 130 million US dollars in 2015 to 1.2 billion US dollars in 2019. The DTx industry is growing swiftly but Europe is at risk of falling behind the DTx markets in places such as the USA, Singapore, Japan and China. For instance, the US Food and Drug Administration (FDA) is proactively encouraging DTx industry through the Digital Health Center of Excellence. Europe currently does not work as a single market nor is there mutual recognition of certification or in many cases even clinical evidence. Currently, Europe is split into over 30 separate markets. Many of these markets do not have a single DTx. Competition between individual countries is not beneficial. Healthcare costs are rising faster than the economy across Europe (PDF). Countries should learn from each other and apply proven tools from other systems to theirs. Success and prosperity for one country does not mean less for another. Furthermore, competition between DTx companies does not truly exist currently as there are only few of them. Without a single market for Europe, there is a risk of falling behind larger countries like the USA or China, leading to a lack of variety and quality in DTx.
European countries have different health systems and care conventions differ between them. However, according to our research and expert views, the differences are small in the criteria they use for evaluating digital health solutions. A good clinical trial in one country is still a good clinical trial in another country. The most important criteria for clinical evidence are not significantly different between countries. Economical assessment and changes to current pathways do differ somewhat between countries, but this is often a result of differences in systems.
Better care and insights on patients
Health systems would benefit from DTx by enabling value- and outcomes-based models of care and self-care, digitalising parts of their operations, releasing resources to other tasks, improving patient engagement, expanding care outside of traditional settings and facilitating prevention. In comparison to conventional therapeutics, DTx can offer possibilities for lower unit costs, thus reducing the costs per patient. The effect depends largely on the pricing and purchasing model but digital tools in general scale up better than drugs or face-to-face interaction and have lower development costs than pharmaceuticals.
Patient-reported outcomes (PRO) are health outcomes reported directly by patients. Most DTx produce PROs and could help them become more central to healthcare. Examples of PROs are quality-of-life measures, symptoms and satisfaction with care. Health systems globally lack these measurements and the reasons for this pose adoption challenges for DTx. These challenges include system-level implementation, system infrastructure inadequacy, and training and engagement of clinical staff. The underlying larger problem is the focus on treating sickness instead of enabling health. One expert from the public sector described the current health systems as “sickness machines” instead of health systems. The focus is not on keeping people healthy but rather waiting until they become sick. DTx will not on its own solve this systemic problem, but it can help alleviate it in specific care pathways.
The full potential of DTx materialises through integration with health systems. If DTx tools remain isolated applications instead of essential parts of a care pathway, their potential will be diminished now and in the future in particular. Changing the systems and truly integrating DTx into care pathways is no easy task. Healthcare organisations are often siloed and split into different functions. DTx rarely fit neatly into the traditional structures.
One DTx solution that aims to turn knee operation in-patients (those staying overnight at a hospital) into out-patients (leaving hospital after treatment without an overnight stay) benefits the hospital by shortening the length of stay in the hospital ward. However, the costs of this service would often be directed to the orthopaedic unit while the savings would materialise in the hospital ward. To truly benefit from DTx, health systems need to enable cross-functional co-operation and to improve the understanding of their own costs.
Personalised healthcare with empowerment
By far the greatest benefit of DTx is the potential to improve patient health. Currently the biggest beneficiaries are people who struggle with chronic diseases or mental health issues. DTx can provide novel therapy options for unmet or undertreated medical needs.
DTx solutions are delivered directly to patients, which enables the skipping of many hurdles in traditional care settings. Instead of being a passive recipient of care, the patient becomes an active participant in their own health. Patients are given control over their own health or at least personalised interventions. Specific to the patient’s needs, the intervention can be engaging, according to their schedule, in the privacy of their own home and accessible with the patient’s own devices.
All the countries surveyed require DTx to be designed with a human-centric approach and to have good usability and accessibility. Even without such requirements, companies in this field need to invest in usability to keep up with the competition.
New business for old and new players
Pharmaceutical companies have many reasons to be interested in DTx applications and the business opportunities they provide. Companies with a focus on DTx specifically have obvious interests in the industry. The development costs of a drug run into the hundreds of millions of dollars with some reaching into billions of dollars. The time to market for a drug is often upwards of 10 years. After the gruelling path to obtain market access for a drug, user data dries up. DTx has the potential to help with all these challenges.
According to our industry experts, the development time of a DTx solution can be as short as three years, including clinical trials. Clinical trials always take time, but digital tools have shorter development cycles. This can help existing pharma companies to diversify their portfolio and also provide access to the field for small to medium-sized companies that previously were excluded because of the cost of product development.
Successful DTx companies must excel in areas where many pharma companies are lacking, such as data analytics, user-centric design and flexible business models. To avoid large investments and lower risks, pharma companies are looking into partnerships. This way pharma companies benefit from the technologies and new capabilities while DTx companies benefit from existing customer base and scale. There is great promise in this model but as it stands there are few market-ready solutions built upon the partnership model.
3. European countries have similar approaches to DTx but lack alignment
European countries have significant similarities in their approaches to digital health. However, they do not have mutual recognition of certification or of evidence. Approaches to digital health are taking form and many questions about value, scalability and economic feasibility still lack answers.
Overview of the health systems
The selected countries/regions all have differences between their health systems. There are three main ways to finance healthcare in Europe. The first is a public taxation-based system, often referred to as the Beveridge model. The second is the public compulsory social insurance model, often known as the Bismarck model. The third model is privately financed voluntary insurance. Germany, Belgium and France follow the Bismarck model while England and Spain follow the Beveridge model. Catalonia is a part of the Spanish healthcare system and receives funding from the Spanish government. Private insurance and out-of-pocket health services exist in all of these countries to a varying degree.
Regardless of the system, healthcare providers can be employed by the public or private sector. The countries differ in practice on how they approach novel treatments. According to some experts, England is strict when it comes to new, innovative treatments while Germany, for instance, is more proactive. In practice, this can manifest itself in a new cancer drug being reimbursed in Germany while not being reimbursed in the England. The underlying philosophy affects the uptake of DTx. An overview of the systems is given as follows.
- In Germany, there are multiple different autonomous insurance funds with both public and private providers. The central government provides the legislative framework and monitoring. The insurance payment is strongly connected to employer. States (Länder) have executive responsibility over the insurance funds. Almost 90% of the population is covered by the social insurance.
- In France, there are public insurance funds, but these funds do not self-manage like their German counterparts. The French government takes care of the operational and financial management.
- In Belgium, healthcare is primarily funded by mandatory public insurance. The federal government regulates and funds most of the healthcare services through the health insurance.
- The Spanish central government funds healthcare that is provisioned by autonomous regions such as Catalonia. The system is based on tax funding rather than insurance.
- The healthcare system of United Kingdom is formed of different publicly funded systems for England, Northern Ireland, Scotland and Wales. There are some differences between these systems. In this paper, England is the examined region. The healthcare system in England (the National Health Service, NHS) is funded directly by taxes.
System-wide strategic policies
All five countries/regions have system-wide strategic programmes to advance digital aspects of healthcare. They focus on core software such as electronic health records (EHRs) and interoperability and on more innovative areas such as eHealth or mHealth. The reasons for choosing one specific focus, like eHealth instead of mHealth, are unclear.
Out of the examined countries/regions, the focus of Germany comes closest to DTx in its definitions and criteria. The Digital Healthcare Act (2019) enables approved apps to be prescribed and the Digital Health Applications Ordinance (2021) establishes the process through which DTx providers can get their solutions assessed, accepted, reimbursed and integrated into the healthcare system. Other countries have more general approaches focused on mHealth or medical devices in general.
Varying legal frameworks with similar contents
Parts of DTx can fit within many different scopes, such as mHealth, eHealth or health apps, and this is demonstrated by the variety of categories DTx can fall under: mHealth for Belgium and Catalonia , digital health apps (DiGA) for Germany, and England uses many different terminologies within the NHS and NICE (National Institute for Health and Care Excellence) with digital health apps and digital health technologies being some examples. France is working towards using DTx terminology (“thérapies numériques”) at the time of writing. All the digital health solutions feature many similarities in the criteria used to define them, which are, or include, the following:
- software-delivered digital applications;
- able to handle medical data and are thus medical devices (excluding Catalonia);
- user-facing with patient-facing functionalities;
- used to prevent, manage or treat a medical disorder or disease;
- supported by clinical evidence;
- supported by cost-effectiveness or socio-economic evidence (level of evidence depending on country);
- able to undertake some analytic processing (in other words, are not just concerned with data collection and display) (excluding Catalonia, which also certifies general health and wellness applications).
As such, any of these frameworks can fulfil the criteria for DTx, as well as for other solutions. The main subtle difference between the countries was the extent to which a DTx focus on prevention was included. Anything aimed at the secondary or tertiary prevention of a particular disease was likely to be included, but general health and well-being apps would not be. Following the definition in this paper, primary prevention would not be included in the scope of DTx as it requires a disease or condition to be diagnosed. However, due to the variety of perspectives taken by the examined countries, many of them can include primary prevention within their scope.
Medical device regulation changes in Europe can make market authorisation more difficult for companies because medical device certification is necessary for market entry for a DTx solution. Under the former Medical Device Directive (MDD 93/42/EEC PDF) framework, a DTx could technically enter the market without trials by virtue of them falling into Class IIa and IIb. The new Medical Device Regulation (MDR 2017/745 PDF) implemented in May 2021 decrees that such devices will need to prove safety, effectiveness and performance requirements for most DTx. Class I devices will be excluded and can self-certify with a notified body, but it is unclear what proportion of DTx would fall within this category. In practice, this will entail more clinical work and a more demanding evaluation process. According to some experts, this will not prove difficult for most companies as they already have extensive clinical evidence to prove the effectiveness of their solutions. However, it will result in more work as all devices approved under the MDD will need to fulfil the criteria of the MDR within five years of initial certification.
Health Technology Assessment (HTA) is a systematic evaluation of a health technology focusing on its direct effects and unintended consequences. Every country has an HTA framework for all health technologies. The aim is to inform decision-making regarding health technologies. HTAs often inform decisions related to reimbursement and coverage by insurers and national health systems, which requires the HTA assessment to include a cost-benefit assessment. Health technologies include pharmaceuticals, devices, ways of working and software used in healthcare. This means that HTAs are always general approaches which can make them ill-suited for DTx. The countries surveyed approach this by having a separate assessment for digital applications, mHealth or digital technologies.
Assessment and certification schemes and process
Before diving into assessment and certification schemes, it should be noted that the definition of DTx makes it clear which frameworks and schemes were examined. Given its definition, DTx would fall under the Medical Device Regulation (MDR) since DTx solutions handle personal health data. All countries have additional requirements relating to risk assessment, safety evidence, data protection, health outcomes impact and health economic implications. In the case of a DTx solution providing a function already addressed by an alternative approved solution, there might be a requirement to demonstrate its competitive advantage, for example more cost savings or better outcomes.
DTx companies in all countries are expected to determine the specific evidence they wish to submit. Varying detail of guidance on the kinds of evidence required is usually available. DTx is a field that hosts a wide variety of possible solutions, making the openness and ambiguity understandable and even appropriate. Companies value complete and precise guidance on the criteria they need to meet. For instance, Germany solves this issue by assigning a case manager to each company to help with their questions in addition to posting the requirements available for everyone. The practicalities and costs for producing the evidence is always borne by the developer. Germany is the only country to offer a provisional fast-track process to gather further evidence while under reimbursement for the service.
How to seize the value of DTx
Assessments and certifications are known as threshold requirements. They make sure that the product is safe, performs and has positive effects. The real challenge is in how to harness the potential value of DTx into real-world value.
Market stimulation is mostly sporadic and non-systematic. All the countries have one-off projects and programmes to support and introduce digital health solutions but comprehensive models that balance value and innovation are lacking. A likely explanation is the wider focus on the cost of healthcare. New products and solutions are seen as additional costs and health systems are guided by budgets.
Germany is the only surveyed country that has a large-scale model for enabling companies to receive approvals and for creating sustainable businesses. The German model allows a company to receive approval and reimbursement for their solution for one year. During this year the company can gather the final evidence for the effectiveness of the DTx solution. It should be noted that most companies already carry out clinical trials before this initial one-year period. This model is still in its infancy and it remains to be seen whether it is cost-effective and adds value. It clearly does encourage the creation of DTx companies as there are over 20 separate DTx solutions in the German directory of digital apps (DiGA) while other countries have one or two approved DTx solutions.
Integration into healthcare systems is not guaranteed through positive assessment in any country. The first easy step to integration is to publish accepted solutions in a registry. All the countries add any accepted solution to a registry, which enhances the reputation of the solution. After this there are different approaches. Insurance-based countries like Belgium, France and Germany integrate the solutions by reimbursing the clinicians prescribing the solutions. The exact model for Catalonia and England is still unclear. For England, it currently depends on the local healthcare providers (Clinical Commissioning Groups or NHS Trusts). Catalonia is currently pursuing a public tender through which prospective DTx solutions could be integrated into the toolbox of healthcare providers through a traditional licensing scheme.
Reimbursement is a strong incentive and it always requires that the DTx is prescribed by a health professional and is selected from among solutions that have had a prior successful positive HTA-type assessment. However, approval and reimbursement are usually separate decisions, sometimes made by separate bodies based on separate applications. Without reimbursement, companies need to negotiate and prove their value with every buyer, of which there may be thousands in a country. The approval and acceptance for reimbursement of a DTx has sometimes been specified to patient profiles (such as the severity of the disease), care organisations (such as a DTx solution only being allowed to be prescribed by a general practitioner) or a single care pathway (such as lung cancer care pathway). Germany is a notable exception, allowing relatively unrestricted pricing of the solution or product by the company for the initial year after which the pricing levels are defined more through price negotiations with the public authority responsible for pricing.
Do products or services need to be adopted or is it that patients and clinicians need to adopt a new way of working? The former is the established and easier approach to digital health solutions. To truly harness the value of digital healthcare, pathways should be designed with patients and clinicians in mind rather than gluing digital health solutions on top of existing care pathways.
Another challenge with existing care pathways is that they are often siloed. Reimbursement for DTx solutions often comes from a specific area of speciality or function within an organisation while the benefits of a solution may be felt across several different sectors. At the extreme end, the payer may receive no direct benefits within their area of responsibility. This is a challenge for many of the countries surveyed. There may be little incentive to develop DTx solutions that work across the siloes since they may be difficult for healthcare organisations to sell.
Data integration and use
Richer data is often mentioned as a positive of digital health. Digital health applications can offer data that conventional health systems lack, like information on patient engagement, real-time data and patient-generated data. However, many countries are struggling with basic infrastructure issues, such as electronic health records (EHRs) and integrating hospital point-of-care systems into other core systems. DTx data is currently difficult to integrate with existing systems and current ways of working.
DTx tools produce valuable information that can be used for use cases beyond the primary use case within a solution. This information can include details on patient engagement, adherence to therapy, progress in therapy, real-world outcomes and patient-generated data. Data from DTx can be integrated into health systems, specifically EHRs, to better treat patients and to also allow for a better allocation of resources, enable value-based healthcare and to understand operations beyond clinical measurements. DTx data can also help with understanding the effectiveness of existing drugs.
In most of the countries surveyed, the approval of a DTx solution includes a requirement to demonstrate interoperability with a national electronic health record or an eHealth platform, with a published list of standards, and sometimes with a specific Application Programming Interface (API). However, it seems that most of the countries are still on a journey towards being able to import the DTx data and to incorporate it as part of the longitudinal health record of each patient. As such, many DTx solutions operate in their own “data bubble”.
None of the countries surveyed have in place a formalised approach to the reuse of data originating from DTx for secondary purposes such as research. There was often an intention for the health system to do so, but there are no clear current use cases for the data. DTx companies are not allowed to commercially use the data they collect from one product in another product.
Is DTx the answer to our problems?
With all the promises of DTx, there remain some challenges to be solved. Prevention is the best way to reduce the costs of healthcare and to improve a population’s health. However, DTx requires diagnosis and prescription by a doctor, which excludes most cases of primary prevention. DTx can prevent a condition from getting worse (secondary prevention) or can help one live better with a condition (tertiary prevention), but preventing a condition from developing to begin with (primary prevention) precludes any diagnosis.
As national approaches mature, demonstrating the effects of DTx can become more difficult. Currently, solutions using DTx are compared to existing care pathways. It is simple enough to show how the current way could change and what the improvement could be. However, if the market becomes saturated, comparisons become more difficult. Should DTx solutions be compared to each other? What happens when the market becomes saturated? What is “generic DTx” (in comparison to “generic drugs”)? How can a clinician decide what DTx is most applicable to a specific scenario? The healthcare providers might come full circle to conventional tendering if the field expands uncontrollably.
Evidence is at the core of DTx. Validating evidence is no simple task, one which many countries are already struggling with. Medical Device Regulation is trusted but the certifications are rarely verified after the initial certification. Many public authorities struggle with resources, risking having falsely marketed solutions enter the market. Another issue with evidence validation is the ever-changing nature of digital solutions. How often should digital health solutions be assessed after market entry? Countries need to place a lot of trust in companies to define the metrics and to provide evidence.
4. Recommendation for national approaches and European alignment
European alignment would benefit everyone in Europe. However, the role of the European Union (EU) is seen as problematic. The digital health industry is advancing quickly and the EU is seen as being too slow to answer all the challenges. It is essential for European countries to share best practices, learn from each other and build structures to allow for mutual recognition of evidence or even complete certification.
The European Commission should build enablers for mutual recognition across Europe
The role of the European Union in enhancing European collaboration is challenging, with some experts viewing the European Commission as being too slow to lead the way for DTx. There is a risk of too much added bureaucracy and prolonged processing times if the EU has too big a role in the certification process. For mutual recognition of evidence and even certification, co-operation between countries is the most attractive option to many experts. There are at least two ways to approach this. The first is for two major countries, such as Germany and France, to mutually recognise each other’s assessments or at least their clinical evidence. This would act as a blueprint for collaboration between other countries. Another alternative is to build upon the examples from other countries that already have similar systems based on a specific model like the one built jointly by the EUnetHTA network.
The European Commission can help Europe to boost DTx in Europe by:
- creating a benchmark for national requirements;
- establishing a minimum set of requirements that are common across Europe to act as a blueprint for countries whose progress is slower and to help align the existing frameworks;
- facilitating evidence (clinical and socio-economic) exchange between countries;
- building a registry of solutions certified by a notified body – such a registry should include information on what criteria are used by each approved DTx solution and the responsibility for maintaining such a registry could be given to existing agencies like the European Medicine Agency or the European Centre for Disease Prevention and Control, or to existing joint action groups such as EUnetHTA;
- consider market access for digital health solutions in other initiatives such as the European Health Data Space (EHDS).
European countries should create national approaches for DTx
European countries already collaborate with each other in many ways within healthcare. Examples of collaboration include the EU Digital Covid Certificate, the exchange of electronic health records across the EU and the cross-border exchange of electronic prescriptions. However, there is room for greater alignment. There is surprisingly little difference in digital health assessment requirements between countries – one expert estimate placed the percentage of similarity at between 80 and 95 per cent. The differences come mostly from the additional requirements (integration into current care pathways, for example) and not from differences in the fundamental requirements such as medical device regulation, clinical evidence, safety or usability. Our recommendations for national approaches to DTx are as follows.
1. Create inclusive national strategies on DTx
Currently, DTx solutions are not part of any national strategy but rather parts of certain innovation programmes or laws. This results in a low uptake of DTx and inefficient DTx processes.
Given the complexity and promise of the field, DTx should be recognised as the spearhead of digital health and should be included in broader digital health strategies. DTx solutions need to be separated from general health and well-being apps and other medical devices. This will facilitate a harmonised and integrated approach in the digital health ecosystem.
2. Create clear public assessment and certification pathways to assess and approve DTx solutions
Normal HTA pathways are rather slow and complicated to navigate. Most importantly, they are general approaches to health technologies. The excellence of DTx should be recognised. HTA pathways for DTx solutions should be established, together with clear guidelines, requirements and information on the process (such as the length of processing and status).
3. Create and publish clear assessment and evaluation requirements balancing innovation and regulation with consideration for other countries
Each European country should define a framework and the criteria for assessing DTx that balances regulation and innovation. Many such frameworks are publicly available from official national frameworks, trade organisations (such as the DTxAlliance) and working groups (like EUnetHTA). Reinventing the wheel would thus be ill-advised.
Regulation needs to balance innovation with regulation. The framework needs to consider the process from the perspective of companies in addition to the regulatory perspective. The key for the framework is transparency and efficiency. Requirements, processing time and status should be clear and visible to the applicant. The process should not take more than a few months at the most. If possible, the path to reimbursement should be explicit. In countries without a strong central authority, the relevant competent regional authorities should work together to clarify the process for each region.
For clinical evidence, improved quality of care and better clinical outcomes are a desirable goal. Clinical trials are an industry standard that work well with DTx. They should form the basis of clinical evidence. DTx companies broadly prefer recognition of excellence but need transparency, efficiency and realism in the assessment. For instance, aligning DTx assessment with Medical Device Assessment helps reduce duplicated efforts. However, DTx solutions fail to achieve their full potential if they remain isolated within existing care pathways. Countries should consider the larger picture with regard to clinical evidence. This is difficult in practice. The first step is to involve the care provider early on and jointly plan the integration of DTx into the current ways of working.
For socio-economic evidence, health systems and healthcare providers need to provide data and support to DTx companies to achieve the best results for all parties. Using general cost estimates is not enough when comparing DTx to the specific care pathway.
For a DTx solution itself, privacy, security, usability, accessibility and interoperability need to be considered, as with any software-based medical product or service. For all of these, industry standards exist and can provide a blueprint for these requirements.
4. Provide a path to market access
DTx solutions come under strict scrutiny. To make it through the whole process, each solution will have had to prove its clinical effectiveness, socio-economic effects, usability and safety, to name just a few of the key requirements. As a result, each should be used in healthcare provision. A clear process for receiving market access needs to be in place. This depends on the model of healthcare financing. Reimbursement for DTx solutions is a more flexible way compared to procurement. However, procurement may be suitable for DTx tools that rely on self-prescription and that are offered to whole population.
5. Facilitate growth of DTx companies by providing access to real-world evidence (RWE)
Even though DTx can have much lower development costs compared to traditional pharmaceutical therapeutics, the costs are still a barrier to entry for smaller companies. DTx solutions also generally have less potential for severe side effects. Countries can encourage DTx companies by providing access to further data to prove the effectiveness of their solutions. This does not imply less strict requirements for the solutions. It is difficult to gather all relevant information through clinical trials since many of the practicalities of health systems are omitted.
The German fast-track process is an example of such a model. This gives DTx companies a year to gather the rest of the evidence for their solution. About three quarters of the companies entering the assessment process select the fast-track option. This helps companies adapt their solution to the system and to serve the needs of the system better.
6. Tackle resistance to change before it appears
Health systems are notorious for their resistance to change. This resistance can never be completely removed but it can be made easier to manage by increasing the understanding of the potential impacts of DTx and by transparently communicating the changes and their implications. Outcomes should be visible to patients, clinicians and care providers. This is a task for the regulators, the payers and DTx companies. Clinicians broadly trust published studies and data on drug effectiveness and treatment risks. Information on the approvals and assessment of DTx solutions should be visible to clinicians and handled in a similar manner.
Patients are expected to adapt quickly to changes introduced by DTx, if they can trust them. Health and wellness apps already play a significant role in mHealth while there is currently a lack of DTx in the healthcare system. One way to enable patients and clinicians to decide on the relevance of a DTx solution for their individual needs would be to filter and compare DTx solutions for specific conditions or within a certain care pathway, based on quality criteria that they can easily understand.
7. Plan the integration of DTx into healthcare systems
DTx solutions risk becoming sporadic solutions without integration into the wider context. This risk is especially apparent with approaches like that in Germany, where the focus is on approving DTx solutions and placing them within the reimbursement scheme. Traditional procurements may not be optimal in terms of facilitating innovation, but they have better prerequisites for integration.
This challenge was recognised by all of the experts in our research but there is no consensus on how to solve it. However, more co-operation between care providers and DTx companies would help to integrate DTx within digital workflows.
8. Use the opportunity for DTx to build value- or outcome-based models of care delivery or procurement
Digital health solutions offer an unprecedented opportunity to rework the fundamentals of health systems, moving away from activity-based costing (payment based on the number of procedures) to value-based (payment based on health outcomes). Value-based models of care could incentivise DTx even without specific reimbursement.
References
Interviews
In total, 15 interviews were held during August and September 2021, with at least one public servant involved with some part of DTx strategy, assessment or reimbursement and one person from the DTx industry per country interviewed. Many interviewees did not agree to disclose their names and organisations and, as a result, no names or organisations are being disclosed.
Workshop
Interviewees and selected experts participated in a workshop to discuss what should be done in Europe. There were around 30 participants in total.
Literary material
Links retrieved on 8 November 2021.
Digital Therapeutics Alliance. Digital Therapeutics Definition and Core Principles (PDF)
European Union (1993). Council Directive 93/42/EEC (PDF)
European Union (2017). Regulation (EU) 2017/745 of the European Parliament and of the Council (PDF)
FDA (2021). Digital Health Software Precertification (Pre-Cert) Program
IQVIA (2021). Digital Health Trends 2021
McKinsey (2020). How COVID-19 has pushed companies over the technology tipping point—and transformed business forever
Moovcare (2021). Clinical studies. The scientific study: Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment
Pitchbook (2020). PitchBook Analyst Note: The Digital Therapeutics Revolution
World Health Organization (2006). Telemedicine, Telehealth, and Health Information Technology (PDF)
World Health Organization (2011). mHealth: new horizons for health through mobile technologies: second global survey on eHealth
World Health Organization (2019). WHO guideline recommendations on digital interventions for health system strengthening (PDF)
World Health Organization (2020). COVID-19 significantly impacts health services for noncommunicable diseases
World Health Organization (2021). Spending on health in Europe: entering a new era (PDF).
Wouters O., McKee M. and Luyten J. (2020). Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018















Recommended
Have some more.